Cr VI (Chromium 6) found in cement is followed by the sector due to its hazards on human health. According to European Parliament 2003/53/EC Directive, Cr(VI) content in cements used and sold in all the member countries is required to be no more than 2 ppm as of 17 January 2005. As of 3 January 2022, following the European Union Directive which prohibits the sales of cements with a content of Cr6+ (Cr VI) over 2ppm after hydration, iron sulphate started to be added into the cements. You can check out Cement Additives: Chromium VI in Cement and Sustainability and Chromium: Cr VI in Cements to learn more.
What is Cr VI?
Chromium, especially chromium VI, has high toxicity. Cr(VI), which can dissolve in cement, is found as dichromate or chromate and it causes diseases especially on the skin such as allergic reactions and eczema.
How Does Cr VI Form?
Chromium in furnace feed is primarily in the form of Cr (III). The conditions inside the furnace contain high amounts of CaO, free lime and alkali originating from internal circulation of volatile materials. Such conditions are suitable conditions for oxidation of chromium to chromium VI and the amount of formation depends on the oxygen pressure inside the furnace environment.
The probable causes having chromium(VI) in clinker and cement may be listed as follows: the total chromium which entered the system through raw material and fuel being oxidized during clinker kiln due to the oxidizing environment in the furnace, usage of chromium magnesite bricks in the furnace as refractory, usage of chromium alloy in breakers and mills in the process and usage of chromium containing materials such as usage of gypsum as setting retarder and pozzolana and blast furnace slag as additives, etc. The Cr(VI) form poses some risks in terms of health and environment since it has high solubility, high oxidizing properties compared to the other forms, and it is more reactive due to its chemical instability. As a result of contact of cement with the skin, Cr(VI) content in the cement penetrates the skin and causes allergic dermatitis.
How To Reduce Cr VI?
Iron (II) sulphate among iron (II) compounds which is used in the reduction of Cr (VI) is an effective compound commonly used in cement industry. It exists in heptahydrate and monohydrate forms. It is produced as a byproduct during iron and steel production and processing as well as TiO2 production and therefore it is an economical reducing agent in terms of price.
Iron (II) ion’s reducing 6 valence chromium to 3 valence chromium occurs according to the following reactions.
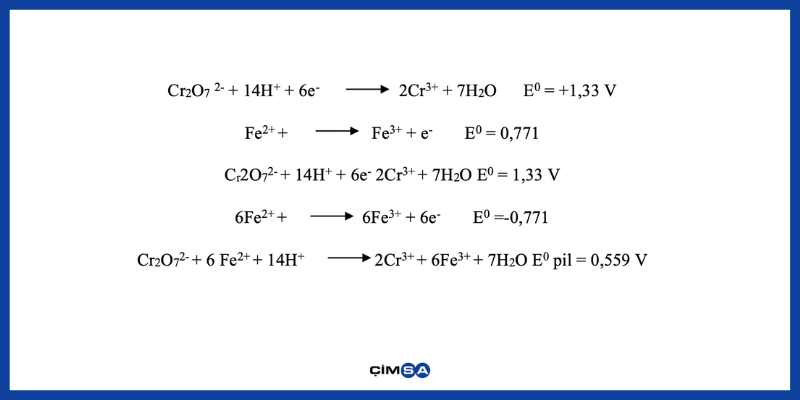
Since E0 pile value is positive, net reaction occurs automatically, in other words, Cr (VI) is reduced to Cr (III) in watery solution with Fe2+ ion. Cr (VI)’s reduction to Cr (III) is dependent on the environment’s pH level, reaction time, Cr (VI) concentration and amount of reducing agent. If iron (II) sulphate is added into the cement during mill grinding and is exposed to the heat generated during cement production, a certain part of Fe2+ ions would oxidize with the effect of heat and humidity to turn into Fe3+ ions and thus would lose their capacity of reducing Cr (VI) ions. In addition, a stoichiometric amount more than needed is required be used to offset the loss that would originate from Fe2+ in order to adjust the desired level of Cr (VI). However, usage of high levels of iron (II) sulphate can cause increases in water demand of cement and prolonged setting times. It would be more beneficial to add iron (II) sulphate at the outlet of the mill and to decrease its exposure to heat in order to eliminate this disadvantage.
What to Know about Cr VI?
Although chromium found in cement is usually in Cr(III) form, it may also be found in different forms such as Cr(VI), Cr(V) and Cr(IV). Cr(VI) form poses some risks in terms of health and environment since it has high solubility, high oxidizing properties compared to the other forms, and it is more reactive due to its chemical instability. Reducing Cr6+ ion in cement extends cement’s shelf life and increases its quality.
References:
- M., C. (2003). Chromium VI in cement, Global Cement and Lime” Environment. 12-13.
- Sanchez, F. M. (2001). “Total and soluble chromium, nickel and cobalt content in the main materials used in the manufacturing of Spanish commercial cements”, Cement and Concrete Research, 435-440.


