In the construction chemicals sector today, there is a requirement of offering different solutions for different demands (high temperature, early strength, fast setting, crack-free structure). As such, ternary systems are created using different ratios of Portland cement (PC), calcium aluminate cement (CAC) and calcium sulphate rather than using only Portland cement to prepare mixtures. Mixture ratios in ternary systems may be renewed depending on the needs. In this post, we will address subjects such as what ternary systems are, what their properties are and where they are used.
What Is Ternary System (OPC- CAC-CaSO4)?
In the below ternary system diagram, the proximity of the points in the triangle to the corners indicates convergence of the composition and characteristic properties to that material.
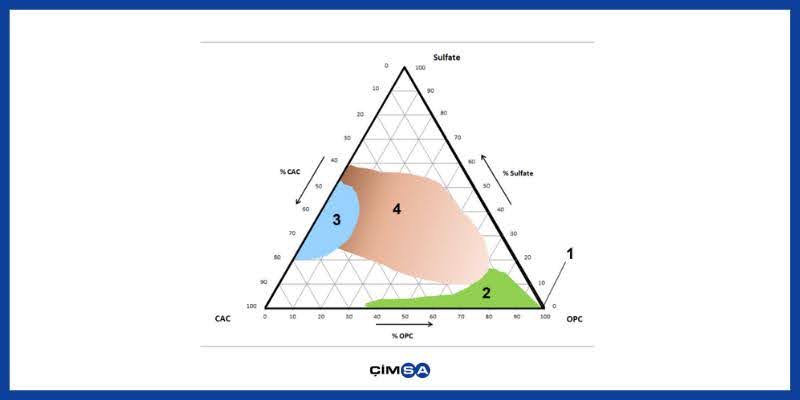
Figure 1. Ternary System Diagram
Zone 2: OPC and CAC Binary System
- Advantage: Fast hardening
It includes OPC and CAC’s binary systems. These types of systems enable just a few minutes of fast setting which is used for repair works in small areas. The advantage of these types of systems is that they only have two components; however, their usage is limited since strengths lower than OPC are obtained from these mixtures and there is no shrinkage offset.
Zone 3; CAC-dominant Ternary System
- Advantage; Early Strength + Fast Drying + Shrinkage Prevention + Alkali Barrier
Transformation from Zone 2 to 3 corresponds to a decrease in OPC content and increase of [CAC + CS*]; this makes ettringite and hydrated alumina the main hydrate phase. In these systems, calcium sulphate is generally added as hemi-hydrate (plaster) or gypsum.
Compared to Zone 2, the properties of systems in Zone 3 related to ettringite formation are improved significantly and early age high strength and fast setting are obtained.
Zone 4; OPC-dominant Ternary System
- Advantage; Early Strength + Shrinkage Prevention
With the increase in CAC ratio, two steps characterize the strength development of the systems. The first step occurring in the first 24 hours is related to the major sedimentation of ettringite which originates from CAC and CS*(plaster) hydration and emerging with OPC phases which can contribute to calcium ions as well. The final strength should be clearly tested in these mixtures.
What are the raw materials of the ternary system (OPC- CAC-CaSO4)?
Portland cement: This product is a material which is used in construction across the world and consumed by far the most after water. Its composition mainly consist of clinker and calcium sulphate. It is made by mixing and cooking clinker, limestone, and clay up to temperatures of 1450°C in rotary furnaces and results with the following phases:
- Alite (C3S): 50% to 70% in mass, and this determined the first hydration. It is the phase that is required for the final strength of the cement and high early strength. If fast cooling does not occur, C3S compounds degrade back to C2S and CaO and alite ratio decreases.
2Ca3SiO5 + 6H2O → 3CaO.2SiO2 .3H2O + 3Ca(OH)2
- Tricalcium aluminate (C3A): 7% to 11% in mass, this is an excessively reactive phase which influences early age hydration kinetics and reacts depending on the presence or absence of calcium sulphate and Ca(OH)2. Having a very fast reaction with water, this phase causes early freezes. Increase in C3A increases early strengths.
- Belite (C2S): enters into reaction with water after 7 to 10 days and it has little contribution to strength in the first days, however it reaches to the same strength value with alite in the long-term.
- Calcium ferroaluminate (C4AF): this is the phase which gives its natural color to cement. It has lower hydration heat compared to other phases.
Calcium aluminate cement (CAC): It is the second component in the amount of OPC-CAC-CaSO4 ternary system. It has been designed to be more durable against sulphate attacks compared to Portland cement, however it is also used due to its properties such as high early strength and high acid neutralization. Currently this cement may be imported from countries such as Türkiye, Spain, Netherlands, United States of America, France, and China. Its usage includes applications such as castable refractory materials, aggressive chemical attacks or corrosive environments and nonstructural components such as final layer coatings. CAC can be compounded with Portland and calcium sulphate depending on the required property for these kinds of applications.
What Are Major Phases?
CA (Monocalcium Aluminate) : This is the most important phase in CAC. In general, 50-55% of clinker is made up by this phase. It is responsible for 24-hour strength in ternary bonding systems. Controlled expansion should be the lower limit for alkali and sulphate resistance.
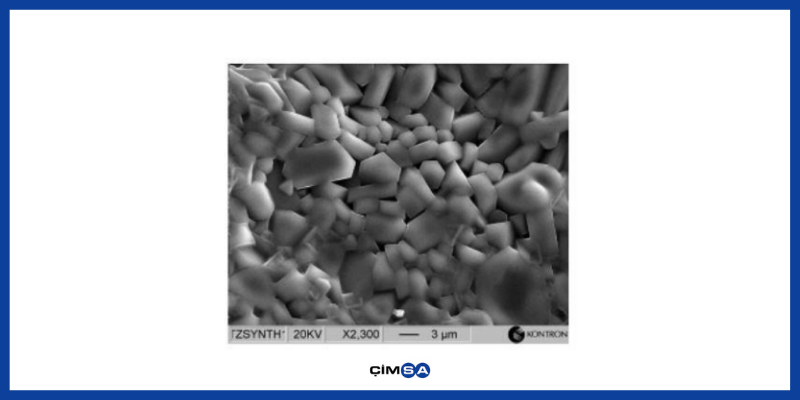
Figure.1 CA Phase SEM Image
C12A7 – Mayenite : It is one of the important phases and plays an active role in setting time. It is the phase that occurs in the presence of humidity. It is responsible for ettringite formation with plaster and workability time in ternary bonding systems. Since it is very reactive, lower, and upper limit values should be determined. High amounts of this phase in cement can lead to uncontrolled expansion. Since it is the material which becomes hydrated first, it can cause an increase in hydration heat in the early period (0-6 hours) and formation of surface cracks.
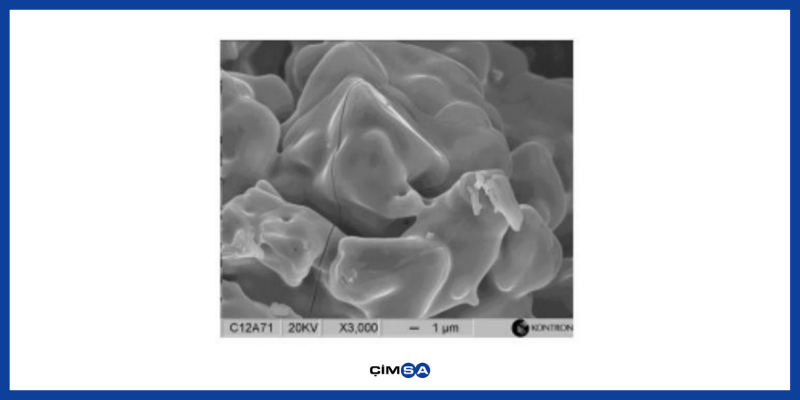
Figure.1 Mayenite Phase SEM Image
Calcium Aluminate Cement Usage in Ternary Systems
CAC is different than Portland cement due to high amounts of alumina (Al2O3) in its chemical compound which can range between 40% to 80%. Highest alumina qualities (70-80%) are obtained from pure raw materials, metallurgic alumina, and lime in rotary furnaces similar to the ones used for the production of Portland cement. Low alumina qualities (40-60%) are obtained from bauxite (its type and iron oxide content determined the color of material which can be gray or black) and limestone through a complete fusion in a ‘reverberatory’ furnace. Calcium aluminate types are based on CA2 ,CA and C12A7 mineralogical components which differ in reactivities. Thus, the lower the ratio of Al2O3 /CaO, the higher its interaction with water and strength. In contrast, the richer it is in terms of component alumina, refractoriness becomes higher, i.e., C12A7 < CA < CA2. Therefore, high alumina calcium aluminates and thus CA2 (decomposition temperature: 1750-1765 °C) are used mostly in refractory applications while in building applications where water reactivity and strength are determinant, cements with lower ratios of Al2O3/CaO should be prioritized. Another determinant factor is the iron content because the higher this value is, the lower its hydraulic reactivity will be and iron containing phase is delayed in a forceful manner with the presence of sulphate anions.
Plaster Usage in Ternary Systems
Another important subject in ternary systems is the reaction rate of the used plaster. This property is directly proportional to the physical and chemical properties of the plaster. Usage of a plaster with slower reactions would create the following effects:
- Low ettringite content
- Low strength
- Low hydration degree
- High permeability
- Low hydrate volume
The weight ratio of crystal water within purse gypsum is 20.90% Partly or completely removing two molecules of water found within the structure of gypsum which constitutes 20.90% of its weight using physical or chemical methods is called dehydration (Gürdal, 1991). Applied physical method is generally increasing temperature by heating.
- Gypsum loses 75% of its crystal water in its core between the temperatures of 95- 165 °C and its weight decreases by 15.68%.
CaSO4 .2H2O CaSO4. l/2 H2O + 3/2H2O (1)
The product obtained at this phase is sulphate semi-hydrate and it constitutes the main material of plaster.
- Gypsum loses all crystal water in its core between the temperatures of 190- 210 °C and its weight decreases by 20.90%.
CaSO4 . l/2H2O CaSO4 + l/2H2O (2)
The product obtained at this phase is called Anhydrite III. Anhydrite III is less plastic than plaster, however it creates a mass which is denser and higher in strength compared to plaster when compounded with water once again under suitable conditions (rehydration). Its characteristic property is very high water-sensitivity. If not isolated, it reacts with the humidity in the air right after it has been produced and turns into semi-hydrate in a very short time.
Where Are Ternary Systems Used?
Portland Cement (PC), Calcium Aluminate Cement-based system applications (CAC) and Calcium Sulphate (CaSO4) are generally limited to interior usage in building areas. The selection of ternary bonds is determined depending on the applications and service life.
Result: Importance and Usage of Ternary Systems
The behavior of ternary hydraulic bonding systems used intensely in construction chemicals sector are also substantially affected in mineral phase ratios of calcium aluminate cement. These mixtures gain mechanical and chemical properties according to their ratios within the ternary system. Although there are various elements in these binders, hydration reactions are shaped according to the ettringites occurring in the presence of aluminum sulphate and calcium in the solution. Early strength and shrinkage preventing property in ternary systems are especially related to the ratio of calcium aluminate cement. Controlling ettringite formation has a direct effect on freeze kinetic, crystal sizes and strength, and the control is required in order to obtain the desired values.
References:
- A Study on the Development of Ternary System Concrete Strength Improving Durability of Concrete . Lee, Dong-Oun: International Journal of Advances in Mechanical and Civil Engineering.
- Calcium Aluminate Cement: Things to Take into Account
- Lamberet, Séverine. 2014. Durability of Ternary Binders Based on Portland Cement, Calcium Aluminate Cement, and Calcium Sulfate. Lausanne, Epfl. 2014.


